Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Tc in radioactive waste arising from research facilities
Tc in radioactive waste arising from research facilitiesKameo, Yutaka; Ishimori, Kenichiro; Hoshi, Akiko; Watanabe, Koichi; Takahashi, Kuniaki
no journal, ,
no abstracts in English
Nishinaka, Ichiro; Nishio, Katsuhisa; Tanikawa, Masashi*; Makii, Hiroyuki; Wakabayashi, Yasuo; Mitsuoka, Shinichi; Yokoyama, Akihiko*
no journal, ,
In order to study the effects of entrance channel mass asymmetry  =(
=(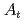 -
-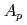 )/(
)/(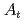 +
+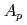 ) on fission fragment anisotropy, we measured fission fragment angular distributions in the reactions of
) on fission fragment anisotropy, we measured fission fragment angular distributions in the reactions of  Ne+
Ne+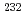 Th (
Th ( =0.827) and
=0.827) and  C+
C+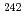 Pu (
Pu ( =0.906), populating the same compound nucleus
=0.906), populating the same compound nucleus 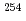 Fm. At the JAEA tandem accelerator fission fragments from the compound nucleus at excitation energies of 56.1, 52.5, 48.8 and 45.2 MeV were measured by position-sensitive parallel plate avalanch counters and silicon surface barrier detectors. The anisotropy for
Fm. At the JAEA tandem accelerator fission fragments from the compound nucleus at excitation energies of 56.1, 52.5, 48.8 and 45.2 MeV were measured by position-sensitive parallel plate avalanch counters and silicon surface barrier detectors. The anisotropy for  Ne+
Ne+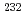 Th with smaller entrance channel mass asymmetry
Th with smaller entrance channel mass asymmetry  =0.827 shows large values compared with those for
=0.827 shows large values compared with those for  C+
C+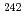 Pu with
Pu with  =0.906. This implies that entrance channel mass asymmetry plays a role for fission fragment anisotropy in the present systems.
=0.906. This implies that entrance channel mass asymmetry plays a role for fission fragment anisotropy in the present systems.
Kokubu, Yoko; Nishizawa, Akimitsu*; Suzuki, Mototaka*; Owaki, Yoshio*; Nishio, Tomohiro*; Matsubara, Akihiro; Ishimaru, Tsuneari
no journal, ,
no abstracts in English
 -ray analysis and behavior of spallation products produced in mercury of the neutron source at J-PARC
-ray analysis and behavior of spallation products produced in mercury of the neutron source at J-PARCKai, Tetsuya; Kasugai, Yoshimi; Oi, Motoki; Wakui, Takashi; Kogawa, Hiroyuki; Haga, Katsuhiro; Hanano, Kohei
no journal, ,
The authors sampled mercury ( 120 g) and the adhesive substances (black powder) from a mercury circulation system in MLF/J-PARC. Twelve nuclides,
120 g) and the adhesive substances (black powder) from a mercury circulation system in MLF/J-PARC. Twelve nuclides, 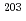 Hg,
Hg,  Pt,
Pt, 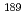 Ir,
Ir,  Ir,
Ir, 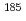 Os,
Os, 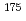 Hf,
Hf, 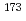 Lu,
Lu, 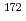 Lu,
Lu, 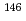 Eu,
Eu, 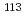 Sn,
Sn, 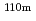 Ag,
Ag,  Y, were identified in the mercury specimen. The amounts of radioactivity were in the range between several to around hundred percents relative to calculation. Only
Y, were identified in the mercury specimen. The amounts of radioactivity were in the range between several to around hundred percents relative to calculation. Only 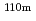 Ag was not found in the adhesive substances. It is expected that other elements than mercury take important role to behavior of the spallation products since the content ratios of the elements were calculated to be in the order of ppb, and the elements (tin and europium) having higher solubility than silver were found in both specimens. For example, iron, which is expected to be a dominant impurity (caused by erosion of stainless steel pipes), dose not react with silver. On the other hand, iron reacts with tin.
Ag was not found in the adhesive substances. It is expected that other elements than mercury take important role to behavior of the spallation products since the content ratios of the elements were calculated to be in the order of ppb, and the elements (tin and europium) having higher solubility than silver were found in both specimens. For example, iron, which is expected to be a dominant impurity (caused by erosion of stainless steel pipes), dose not react with silver. On the other hand, iron reacts with tin.
Kitatsuji, Yoshihiro; Kimura, Takaumi; Kihara, Sorin*
no journal, ,
Based on the electrocatalytic reduction, development of rapid and selective electrolytic reduction of Np(V) to Np(IV) is expected. A flow-through electrode of glassy carbon (GC) fiber platinized by electrodeposition was examined in the present study. Although Np(V) was reduced to Np(III) at the much negative potential at a flow-through electrode of normal GC, stepwise reductions of Np(V) to Np(IV) and Np(IV) to Np(III) were observed on the potential-current relation of electrolysis at platinized GC electrode. The overpotential of reduction of Np(V) to Np(IV) was decreased. This electrolysis method enables us to prepare Np(IV) rapidly without producing Np(III). Np(V) in the mixture solution of Np and U was also reduced to Np(IV) selectively, not accompanied with reduction of U(VI).
Miyashita, Sunao; Kitatsuji, Yoshihiro; Kimura, Takaumi
no journal, ,
The stability constants of trivalent minor actinides (An ;
;  Am and
Am and  Cm) and lanthanides (Ln
Cm) and lanthanides (Ln ; Eu) with Ammonium pyrrolidinedithiocarbamate (APDC) in aqueous solution were investigated. The stability constants were obtained by back-extraction technique using di(2-ethylhexyl)phosphoric acid (HDEHP) as follows. Aqueous phase was acetic buffer solution from 0.02 to 0.10 mol/cm
; Eu) with Ammonium pyrrolidinedithiocarbamate (APDC) in aqueous solution were investigated. The stability constants were obtained by back-extraction technique using di(2-ethylhexyl)phosphoric acid (HDEHP) as follows. Aqueous phase was acetic buffer solution from 0.02 to 0.10 mol/cm containing various concentrations of APDC. Organic phase was toluene or benzene solution containing HDEHP and trace amounts of the extracted radionuclide such as
containing various concentrations of APDC. Organic phase was toluene or benzene solution containing HDEHP and trace amounts of the extracted radionuclide such as  Am or
Am or  Cm. Eqvolumes of the aqueous phase and the organic phase were mixed in vial and were shaken 30 minutes at 298
Cm. Eqvolumes of the aqueous phase and the organic phase were mixed in vial and were shaken 30 minutes at 298  . The results show that minor actinides strongly interacted with dithiocarbamate. From those results, the obtained stability constants (
. The results show that minor actinides strongly interacted with dithiocarbamate. From those results, the obtained stability constants (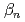 ,
,  = 1, 2) for Am are log
= 1, 2) for Am are log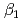 (Am) = 2.5
(Am) = 2.5  0.3, log
0.3, log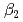 (Am) = 4.3
(Am) = 4.3  0.2 at 298
0.2 at 298  .
.
Hatsukawa, Yuichi; Osawa, Takahito; Appel, P. W. U.*; Arcilla, C.*; Perez, E.*
no journal, ,
Small scale gold mining is widely seen in many places in the world. During gold extract processing, large amount of mercury was released in the environment. Extrapolated to the whole country 200 to 500 tons of mercury is released to the environment every year. Most of the mercury will end in the mangrove swamps fringing the coast line. The mangrove swamps serve as hatching grounds for fish and shellfish the main source of proteins for the Filipinos. Very high concentrations of mercury have been found in water which was used for watering rice paddies. In this study, we developed determination of mercury and gold in the crushed rock mines nondestructively using PGAA and INAA. Also based on the concentration of gold and mercury in tailings, we will optimize conditions of the state battery method. Observed mercury and gold concentrations in tailings are 2.5-393 ppm, 1-3.9 ppm, respectively. Based on these results, we will optimize the conditions of the state battery method for mercury clean up project.
Miyamoto, Yutaka; Yasuda, Kenichiro; Magara, Masaaki; Kimura, Takaumi
no journal, ,
The sequential separation technique of trace U, Th, Pb and lanthanides with a single anion-exchange column has been developed. The pressurized sequential separation system with compressed air was examined to make up an automated separation system. The separation was optimized for some parameters including the flow rate of eluents and the composition of eluents. This pressurized separation system improved the resolution of separation peaks and reduced the separation time compared with the previous gravity flow separation.
Miyamoto, Yutaka; Esaka, Fumitaka; Magara, Masaaki; Kimura, Takaumi
no journal, ,
For accurate analyses of Pu in a Pu oxide/MOX particle, the sample should be chemically separated from Am and U. These interferences lead to systematic error in the quantitative and isotopic analyses of Pu. Two types of chemical separation technique of U, Pu, and Am were examined. One is an anion-exchange separation, and the other is an extraction separation with the TRU resin. Plutonium, U, and Nd which is analogous to Am in the chemical behaviors were successfully separated with the both an anion-exchange column and a TRU extraction column.
 SO
SO /HNO
/HNO mixed solution ([H
mixed solution ([H ] = 1.0 M)
] = 1.0 M)Li, Z.; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Sato, Nozomi; Kikuchi, Takahiro; Sch del, M.*; Nagame, Yuichiro; Liang, X. H.*; et al.
del, M.*; Nagame, Yuichiro; Liang, X. H.*; et al.
no journal, ,
Cation-exchange behavior of  Rf (T
Rf (T = 78 s) produced in the
= 78 s) produced in the  Cm(
Cm( O, 5n) reaction was studied on a "one-atom-at-a-time" scale in H
O, 5n) reaction was studied on a "one-atom-at-a-time" scale in H SO
SO (0.15-0.69 M)/HNO
(0.15-0.69 M)/HNO mixed solutions ([H
mixed solutions ([H ] = 1.0 M) using an automated ion-exchange separation apparatus coupled with the detection system for alpha-spectroscopy. It was found that adsorption probability (%ads) of
] = 1.0 M) using an automated ion-exchange separation apparatus coupled with the detection system for alpha-spectroscopy. It was found that adsorption probability (%ads) of  Rf on cation-exchange resin decreases with increasing [HSO
Rf on cation-exchange resin decreases with increasing [HSO
 ], showing a successive formation of its sulfate complexes. Rf exhibited much weaker formation of the complexes than the lighter homologues Zr and Hf, which is qualitatively in good agreement with theoretical calculations including relativistic effects.
], showing a successive formation of its sulfate complexes. Rf exhibited much weaker formation of the complexes than the lighter homologues Zr and Hf, which is qualitatively in good agreement with theoretical calculations including relativistic effects.
 -HiB complexation and ion-exchange behavior of the group-4 elements as homologues of Rf
-HiB complexation and ion-exchange behavior of the group-4 elements as homologues of RfKikuchi, Takahiro; Toyoshima, Atsushi; Li, Z.; Tsukada, Kazuaki; Asai, Masato; Sato, Tetsuya; Sato, Nozomi; Nagame, Yuichiro; Kasamatsu, Yoshitaka*; Fan, F.*
no journal, ,
We studied complexation of the group-4 elements Zr and Hf as well as the pseudo group-4 element Th with  -hydroxyisobutyric acid (
-hydroxyisobutyric acid ( -HiB) as homologues of Element 104, rutherfordium (Rf). The isotopes of
-HiB) as homologues of Element 104, rutherfordium (Rf). The isotopes of  Zr and
Zr and 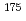 Hf were produced at the JAEA tandem accelerator while
Hf were produced at the JAEA tandem accelerator while  Th was separated from
Th was separated from  U. These radiotracers stocked in
U. These radiotracers stocked in  -HiB/HNO
-HiB/HNO were mixed with cation-exchange resin. After shaking the sample for a certain time, an aliquot of the aqueous phase was precisely taken, which were subjected to
were mixed with cation-exchange resin. After shaking the sample for a certain time, an aliquot of the aqueous phase was precisely taken, which were subjected to  -spectrometry. The complex formation of Zr and Hf reached to chemical equlibria at 180 min. Distribution coefficients of Zr and Hf were decreased with an increase of the concentration of
-spectrometry. The complex formation of Zr and Hf reached to chemical equlibria at 180 min. Distribution coefficients of Zr and Hf were decreased with an increase of the concentration of  -HiB, indicating the consecutive complexation of these ions with
-HiB, indicating the consecutive complexation of these ions with  -HiB. On the other hand, Th was adsorbed on the cation-exchange resin under the given conditions, showing clearly different behavior of Th from that of Zr and Hf.
-HiB. On the other hand, Th was adsorbed on the cation-exchange resin under the given conditions, showing clearly different behavior of Th from that of Zr and Hf.
 Bk and
Bk and 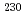 Am
AmKaji, Daiya*; Haba, Hiromitsu*; Kasamatsu, Yoshitaka*; Kudo, Yuki*; Morimoto, Koji*; Morita, Kosuke*; Ozeki, Kazutaka*; Sumita, Takayuki*; Yoneda, Akira*; Koura, Hiroyuki; et al.
no journal, ,
no abstracts in English
Takayama, Reona*; Oe, Kazuhiro*; Komori, Yukiko*; Fujisawa, Hiroyuki*; Kuriyama, Ai*; Kikutani, Yuki*; Kikunaga, Hidetoshi*; Kasamatsu, Yoshitaka*; Yoshimura, Takashi*; Takahashi, Naruto*; et al.
no journal, ,
The extraction behavior of trivalent actinides (Am, Cm, Cf, Es, and Fm) into di(2-ethylhexyl)phosphoric acid, HDEHP, in benzene from HNO was studied together with lanthanides. The extraction constants,
was studied together with lanthanides. The extraction constants, 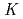
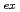 values, of lanthanides, Am, Cm, Cf, and Es were determined by a batch method using stable lanthanide isotopes and radiotracers of
values, of lanthanides, Am, Cm, Cf, and Es were determined by a batch method using stable lanthanide isotopes and radiotracers of  Am,
Am,  Cm,
Cm,  Cf, and
Cf, and 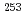 Es, respectively, while that of Fm was measured with
Es, respectively, while that of Fm was measured with 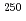 Fm which was produced in the
Fm which was produced in the  U(
U(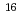 O,4n) reaction and was on-line supplied by a gas-jet method at the AVF cyclotron of RCNP, Osaka University. The
O,4n) reaction and was on-line supplied by a gas-jet method at the AVF cyclotron of RCNP, Osaka University. The 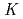
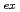 values were determined by the slope analysis for the variation of the distribution ratio as a function of the concentration of HDEHP. We found that the
values were determined by the slope analysis for the variation of the distribution ratio as a function of the concentration of HDEHP. We found that the 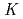
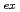 of Fm is smaller than that of Dy in spite of the similar ionic radii while those of other actinides are almost the same as those of each lanthanide with a similar ionic radius.
of Fm is smaller than that of Dy in spite of the similar ionic radii while those of other actinides are almost the same as those of each lanthanide with a similar ionic radius.
 Lu for radioimmunotherapy
Lu for radioimmunotherapyHashimoto, Kazuyuki; Watanabe, Satoshi; Ishioka, Noriko
no journal, ,
no abstracts in English
 Re-MAG3 and
Re-MAG3 and  Re-[(CO)
Re-[(CO) (H
(H O)
O) ]
]
Kurihara, Yuichi*; Nogawa, Norio*; Hashimoto, Kazuyuki; Koike, Yuya*; Morikawa, Naotake*; Ijiri, Kenichi*
no journal, ,
no abstracts in English
 W-
W- Re generator using PZC
Re generator using PZCKurihara, Yuichi*; Nogawa, Norio*; Hashimoto, Kazuyuki; Koike, Yuya*; Morikawa, Naotake*; Ijiri, Kenichi*
no journal, ,
no abstracts in English
 mixed solutions; Towards the study of ion-exchange behavior of
mixed solutions; Towards the study of ion-exchange behavior of 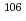 Sg
SgLiang, X.; Li, Z.; Tsukada, Kazuaki; Toyoshima, Atsushi; Asai, Masato; Sato, Tetsuya; Sato, Nozomi; Kikuchi, Takahiro; Kaneya, Yusuke; Nagame, Yuichiro
no journal, ,
Anion-exchange behavior of the group-6 elements, W and Mo, in HF and HNO mixed solutions has been studied by a batch method using the carrier-free radiotracers
mixed solutions has been studied by a batch method using the carrier-free radiotracers 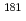 W and
W and 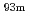 Mo. The long-lived isotope
Mo. The long-lived isotope  W and the short-lived isotope
W and the short-lived isotope 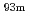 Mo were produced in the
Mo were produced in the  Ta(p,n) and the
Ta(p,n) and the 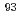 Nb(p,n) reaction at the JAEA tandem accelerator, respectively. The solutions containing
Nb(p,n) reaction at the JAEA tandem accelerator, respectively. The solutions containing 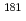 W or
W or 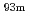 Mo were mixed with anion exchange resin. Radioactivities of the solution were assayed for
Mo were mixed with anion exchange resin. Radioactivities of the solution were assayed for  -ray spectrometry, and then distribution coefficients (K
-ray spectrometry, and then distribution coefficients (K s) of these elements were determined. It was found that the K
s) of these elements were determined. It was found that the K values of W depend strongly on the concentration of HF, while those of Mo are almost constant as a function of [HF] under the given conditions.
values of W depend strongly on the concentration of HF, while those of Mo are almost constant as a function of [HF] under the given conditions.
 SO
SO solutions
solutionsIkarashi, Satoshi*; Sueki, Keisuke*; Tsukada, Kazuaki; Nagame, Yuichiro
no journal, ,
The adsorption rates of Zr and Hf on the cation-exchange resin in H SO
SO solutions were investigated in order to lead to the quantitative discussion of the chromatography. It was obtained the adsorption rates of
solutions were investigated in order to lead to the quantitative discussion of the chromatography. It was obtained the adsorption rates of  Zr and
Zr and 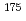 Hf on the cation-exchange resin in 0.1 M H
Hf on the cation-exchange resin in 0.1 M H SO
SO at 15, 25 and 45
at 15, 25 and 45 C as functions of the shaking times. The results showed that the equilibrium of cation-exchange was reached at 70
C as functions of the shaking times. The results showed that the equilibrium of cation-exchange was reached at 70 80 seconds and that the adsorption rates on the cation-exchange resin were not changed between 15
80 seconds and that the adsorption rates on the cation-exchange resin were not changed between 15 45
45 C in the present condition. In H
C in the present condition. In H SO
SO /HNO
/HNO mixed solution ([H
mixed solution ([H ]
]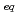 = 1.0 M), the adsorption rate was similar to those of
= 1.0 M), the adsorption rate was similar to those of  Zr and
Zr and 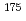 Hf on the cation-exchange resin in H
Hf on the cation-exchange resin in H SO
SO solution.
solution.
Sato, Nozomi; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Toyoshima, Atsushi; Li, Z.; Kikuchi, Takahiro; Kaneya, Yusuke*; Ichikawa, Shinichi; Nagame, Yuichiro; et al.
no journal, ,
It is important to determine the first ionization potentials (IPs) of the heaviest elements with atomic number Z  100 to understand their valence electronic structure affected by relativistic effects. Due to low production rates and short half-lives of the heaviest nuclides, IPs of these elements have not been so far determined. We are developing a surface-ionization-type ion source coupled to a gas-jet transport system in the Isotope Separator On-Line (ISOL) at the JAEA tandem accelerator facility to measure the IP of the heaviest actinide, lawrencium (Lr, Z = 103). In the symposium, we report the present status of the development of the apparatus. Production yield measurements of
100 to understand their valence electronic structure affected by relativistic effects. Due to low production rates and short half-lives of the heaviest nuclides, IPs of these elements have not been so far determined. We are developing a surface-ionization-type ion source coupled to a gas-jet transport system in the Isotope Separator On-Line (ISOL) at the JAEA tandem accelerator facility to measure the IP of the heaviest actinide, lawrencium (Lr, Z = 103). In the symposium, we report the present status of the development of the apparatus. Production yield measurements of  Lr (half-life 27 s), that is suitable for this study, will be also presented.
Lr (half-life 27 s), that is suitable for this study, will be also presented.
 Sg via the
Sg via the  Cm(
Cm( Ne,5
Ne,5 )
) Sg reaction
Sg reactionHaba, Hiromitsu*; Oe, Kazuhiro*; Ozeki, Kazutaka*; Kasamatsu, Yoshitaka*; Kaji, Daiya*; Kikunaga, Hidetoshi*; Kudo, Hisaaki*; Kudo, Yuki*; Komori, Yukiko*; Sato, Nozomi; et al.
no journal, ,
RIKEN group has investigated the performance of the system using an isotope of element 106,  Sg, produced in the
Sg, produced in the  Cm(
Cm( Ne,5
Ne,5 ) reaction. Alpha-particles of
) reaction. Alpha-particles of  Sg separated with a GAs-filled Recoil Ion Separator (GARIS) and extracted to a chemistry laboratory were successfully identified with a rotating wheel system for
Sg separated with a GAs-filled Recoil Ion Separator (GARIS) and extracted to a chemistry laboratory were successfully identified with a rotating wheel system for  -spectrometry under low background conditions. As a result, two isomeric states in
-spectrometry under low background conditions. As a result, two isomeric states in  Sg,
Sg, 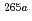 Sg (
Sg ( -particle energy
-particle energy 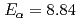 MeV, half-life
MeV, half-life 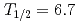 s) and
s) and 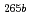 Sg (
Sg (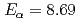 MeV,
MeV, 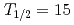 s), were identified. The cross sections for the
s), were identified. The cross sections for the  Cm(
Cm( Ne,5
Ne,5 ) reactions at 118 MeV were roughly evaluated to be 200 pb for
) reactions at 118 MeV were roughly evaluated to be 200 pb for 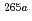 Sg and 170 pb for
Sg and 170 pb for 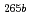 Sg.
Sg.